Amyloid deposition and neighboring tissue responses remain poorly understood. Patient series have shown that with Congo red staining of marrow biopsies about 20% of AL patients have interstitial deposits, 40% have vascular and 40% have no amyloid seen and that marrow amyloid is rare in patients with myeloma. (Ann Hematol. 2011;90(1):101-6; Am J Clin Pathol. 2003;120(4):610-6) The constituents of the deposits have been summarized for 65 AL biopsies, a third with extensive interstitial deposits; apolipoproteins E, A-IV and J are prominently present in the examples offered as well as in an extensive summary of the amyloid proteome.(Amyloid. 2022;29(3):156-64; Crit Rev Biochem Mol Biol. 2021;56(5):526-42) With the goal of achieving a better understanding of the impact AL amyloid deposits might have on the marrow microenvironment we undertook a systematic assessment, within the limitations of specimen availability and quality, of the CD138-depleted marrow mononuclear cells (MNC) and marrow and blood plasma from AL patients with (+MA) and without (-MA) interstitial marrow amyloid. Data on the 49 consenting AL patients are archived in the Harvard Dataverse (https://doi.org/10.7910/DVN/TDFMAI). We compared +MA and -MA CD138-depleted MNC from AL patients by gene expression profiling (GEP) on the Illumina platform; reads were mapped to the UCSC hg19 human genome with Tophat 2/Bowtie 2. Normalization and differential expression analyses were performed with Cuffdiff.(Methods Mol Biol. 2016;1374:339-61) We also compared a different set of +MA and -MA CD138-depleted MNC by single-cell RNA-sequencing (scRNA seq) employing the 10X Genomics Chromium Single Cell 3′ method in our core facility and processing the samples for evaluation through CellRanger, Seurat v. 4.0.3 and the Azimuth marrow reference-based method at the 31 cell type resolution. In addition, we compared peripheral blood and marrow plasma from these patients by immunoprecipitation and immunoblot with respect to apolipoproteins and free light chains (FLC) in complex with the erythroid protein TMCC2 and by ELISA for marrow apolipoprotein E and J levels.
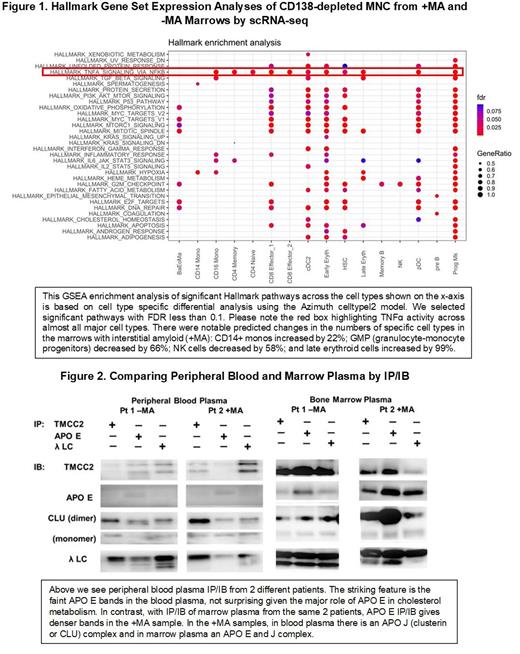
GEP showed no differential expression of genes for proteolytic enzymes or apolipoproteins between the groups but +MA cases had significantly up-regulated erythroid genes involved in oxygen transport, including transmembrane and coiled-coil domain family 2 ( TMCC2). In +MA marrows at the single-cell level, CD14+ monocytes were increased by 22%, granulocyte-monocyte progenitors decreased by 66%, and erythroid-megakaryocyte progenitors and early and late erythroid progenitors increased up to five-fold. Gene enrichment studies showed that in +MA marrows pathways for TNFα signaling via NFκ-B were significantly active (Figure 1); immune activation and monocyte hypoxia were also significantly enriched. As shown in Figure 2, apolipoprotein J is strongly associated with FLC in blood and E is not, while in +MA marrow plasma apolipoproteins J and E are strongly present in association with FLC. By ELISA, there is also significantly more apolipoprotein E and J in +MA marrow plasma. Our data suggest that erythroid-related activity is enhanced in marrows with interstitial amyloid and that a red blood cell related protein, TMCC2, is associated with apolipoproteins and FLC, possibly providing a loose cargo of chaperones that may abet fibril formation by FLC with predisposed secondary structures whose fragmentation sites are made optimally accessible by the loose complex; sites in the FR4 and 5' constant region of AL FLC may be highly vulnerable if exposed in such fashion to perivisceral proteases associated with matrix remodeling.
The marrow microenvironment is clearly impacted by the presence of interstitial amyloid. In HSC from +MA marrows there is increased signaling in pathways that affect lineage commitments such as TNF-α/NF-κB and PI3 kinase/AKT/mTOR. In monocytes from +MA specimens there is evidence of modulation of NF-kB. Both alterations are possibly due to apolipoprotein E, although there is no evidence that monocyte transcription of APO E in +MA CD138-depleted marrow MNC is increased. In conclusion, marrows with interstitial amyloid provide opportunities to study amyloid's impact on cellular behavior, regenerative activity and chaperone involvement in amyloid deposition.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal